marketing@ikrispharmanetwork.com - GST NO. : 09AADCI6001P1ZD
- Send Email
| Business Type | Manufacturer, Exporter, Supplier |
| Specification PDF | |
| Specification PDF | |
| Shelf Life | 24 Months |
| Click to view more | |
Product Details
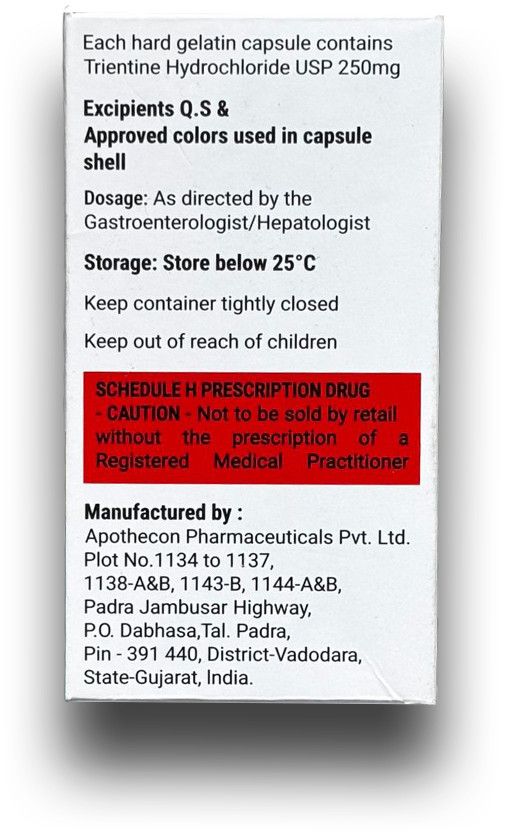
Trientine Hydrochloride 250 mg Capsules, is a generic version of Trientine Hydrochloride 250 mg, developed and manufactured in a USFDA-approved facility by Ikris Pharma Network Pvt. Ltd., India. This medicine is available in India for the management of Wilson’s Disease, a rare genetic disorder that causes toxic copper accumulation in the most important organs, like the liver, brain, and eyes.
Triokris binds excess copper and enhances its urinary excretion, helping restore copper balance and prevent long-term organ damage. It is widely recognized as a safe and effective alternative to D-Penicillamine, suitable for long-term therapy.
Dosage and Administration:
- Adults (≥13 years): The initial dose is 750 to 1250 mg per day, divided into two, three, or four doses daily.
- Children (≤12 years): The initial dose is 500 to 750 mg per day, given in divided doses two to four times daily.
- Maximum dose: Up to 2000 mg/day for adults and 1500 mg/day for pediatric patients aged 12 years or under.
Administration Guidelines:
- Take Trientine Hydrochloride Capsules on an empty stomach, at least one hour before meals or two hours after meals.
- Maintain at least a one-hour gap between Triokris and any other medication, food, or milk.
- Capsules should be swallowed whole with water — do not open or chew the capsules.
- Maintain consistent dosing times each day to ensure steady copper control and effective treatment results.
Clinical Evidence:
- Trientine 250 mg is a proven copper chelator for Wilson’s disease, effective in both hepatic and neurological forms.
- In comparative studies, efficacy was similar to D-penicillamine for improvement or stabilization of hepatic and neurological symptoms.
- Better tolerability — discontinuation rates were significantly lower with trientine (7.1%) than with penicillamine (28.8%).
- Non-inferior maintenance efficacy demonstrated in the CHELAT trial, where trientine maintained copper control equivalent to penicillamine in stable patients.
- Well-established long-term safety, making it the preferred alternative in patients intolerant to penicillamine or zinc therapy.